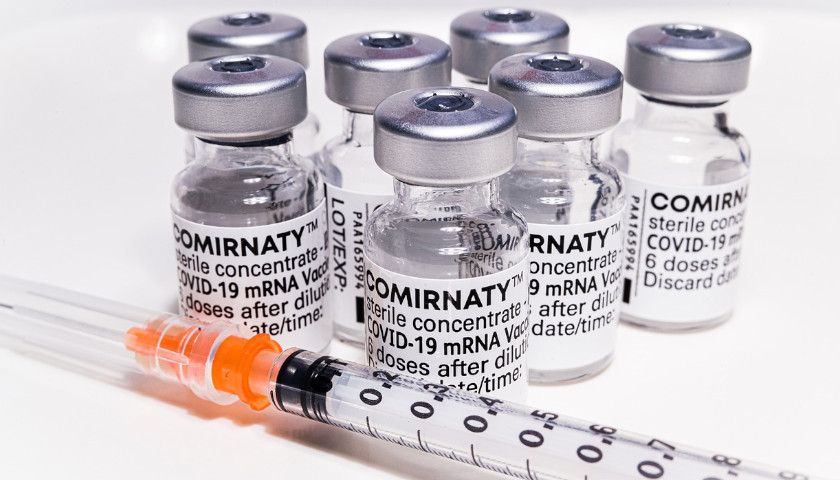
New York-based Pfizer has sold and shipped hundreds of millions of doses of its Food and Drug Administration (FDA) approved COVID-19 vaccine Comirnaty to the European Union (EU) despite saying last week that it is not being shipped in the United States.
“Pfizer Inc. (NYSE: PFE) and BioNTech SE (Nasdaq: BNTX) today announced they will supply an additional 100 million doses of COMIRNATY®, the companies’ COVID-19 vaccine, to the 27 European Union (EU) member states in 2021,” Pfizer said in an April press release. “This announcement is a result of the European Commission’s (EC) decision to exercise its option to purchase an additional 100 million doses under its expanded Advanced Purchase Agreement signed on February 17, 2021. This brings the total number of doses to be delivered to the EU to 600 million.”
Comirnaty was approved by the FDA in August.
The company did not respond to The Star News Network’s request for comment Wednesday.
It has said that it will continue to ship the Emergency Use Authorization (EUA) version of its vaccine in the United States until the supply runs out or expires.
In several places, this policy has caused legal issues.
The Ohio Star reported Tuesday that along with the University of Cincinnati and the University of Ohio, Miami University in Ohio is being sued for over its vaccine mandates.
In those cases, lawyers are challenging the validity of the vaccine mandates based on an Ohio law that took effect in October banning public universities from imposing vaccine mandates for non-FDA-approved vaccines. Since those FDA-approved vaccines are not being shipped or distributed in the United States, attorneys argue that the universities are in violation of the law.
Similarly, a federal lawsuit by military members in the Northern District of Florida, which was eventually thrown out on different grounds, said the Department of Defense’s (DOD) vaccine mandate was also invalid because Comirnaty was not available in the U.S.
The DOD argued that the EUA version of the vaccine was interchangeable with Comirnaty, but Judge Allen Winsor didn’t buy that argument.
“In short, what people think of as the Pfizer vaccine has two distinct FDA approval statuses,” Winsor wrote in his ruling. “It is licensed—that is, fully approved—for the two-dose application in those 16 and older. But it is unlicensed and operating under an EUA— that is, an emergency use authorization—for other applications, like for children under 16 and for certain third shots. Nonetheless, the FDA describes the two as the ‘same formulation’ and ‘interchangeabl[e]’ for medical purposes.”
“Notably, though, the plaintiffs have shown that the DOD is requiring injections from vials not labeled ‘Comirnaty,’” he wrote later in the ruling. “Indeed, defense counsel could not even say whether vaccines labeled ‘Comirnaty’ exist at all. (Although the DOD’s response said it had an adequate Comirnaty supply, it later clarified that it was mandating vaccines from EUA-labeled vials…).”
He went on to call the DOD’s argument “unconvincing.”
It remains unclear when Pfizer’s Comirnaty vaccine will be available in the United States. The company has not responded to multiple inquiries on that front.
– – –
Pete D’Abrosca is a reporter at The Star News Network. Follow Pete on Twitter. Email tips to [email protected].